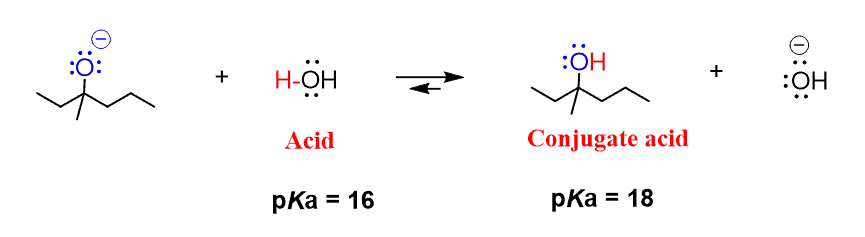
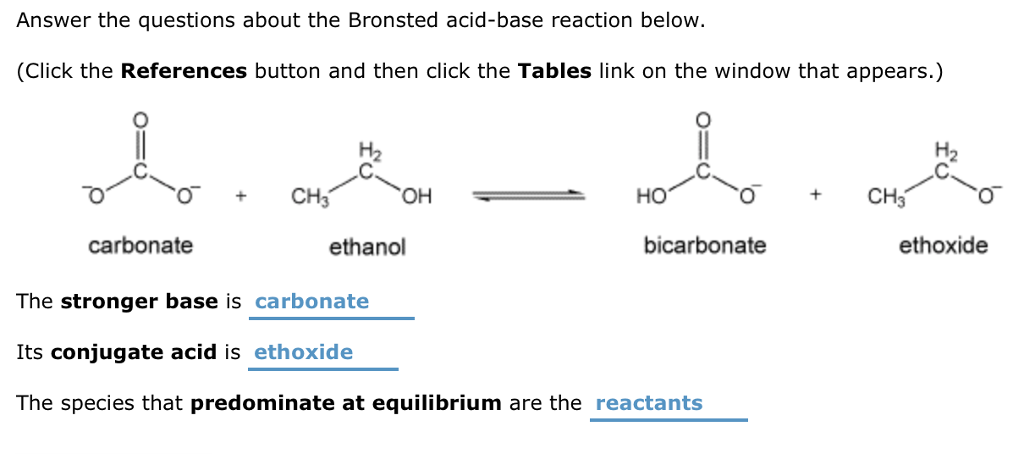
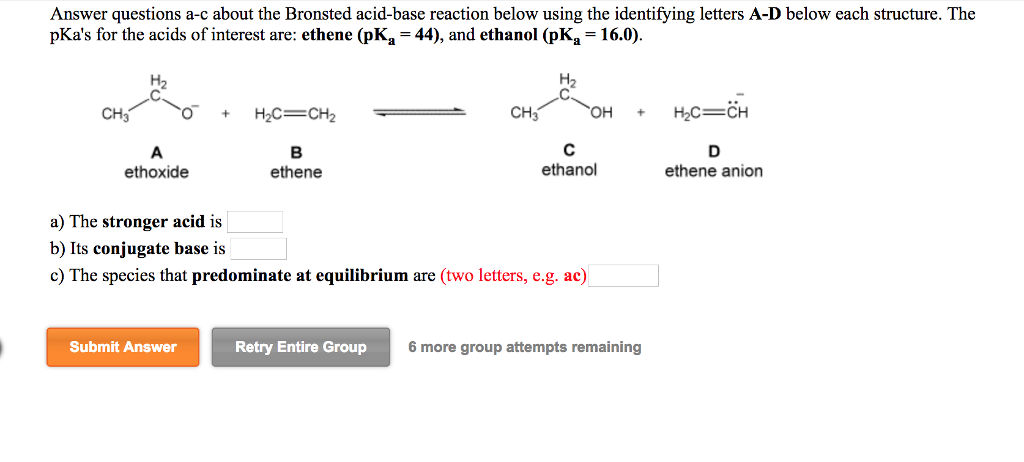
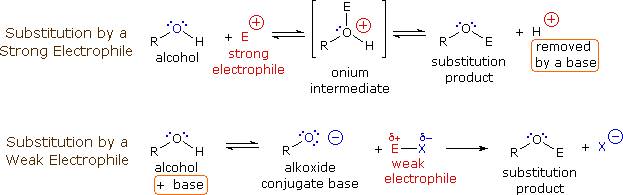
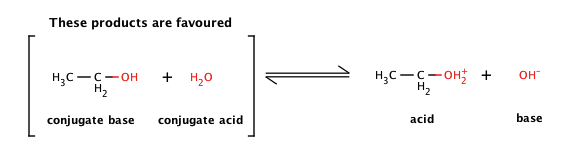
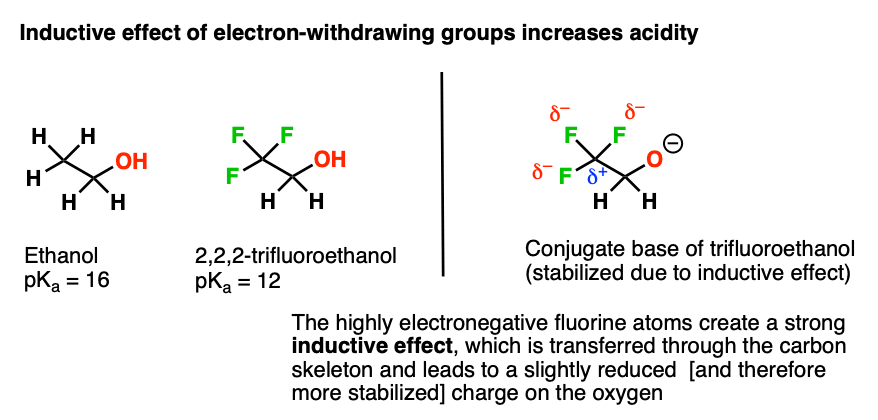
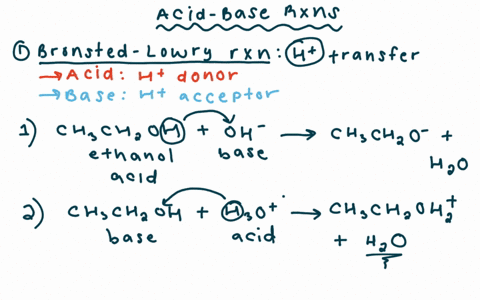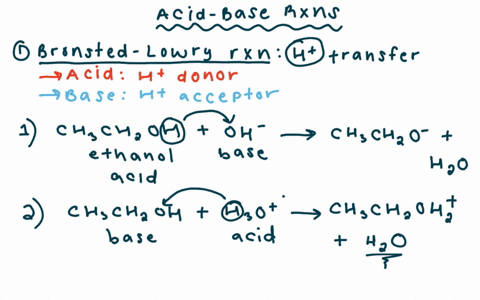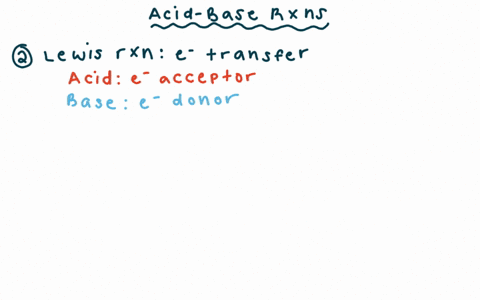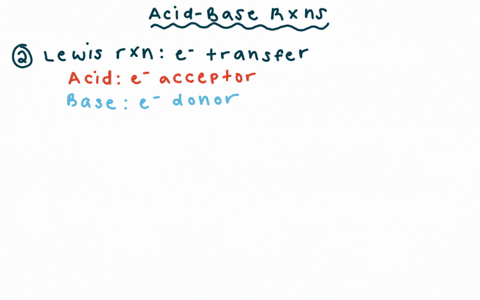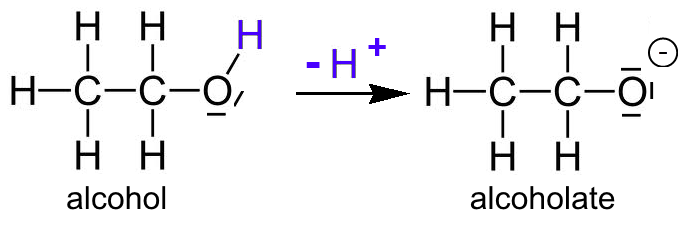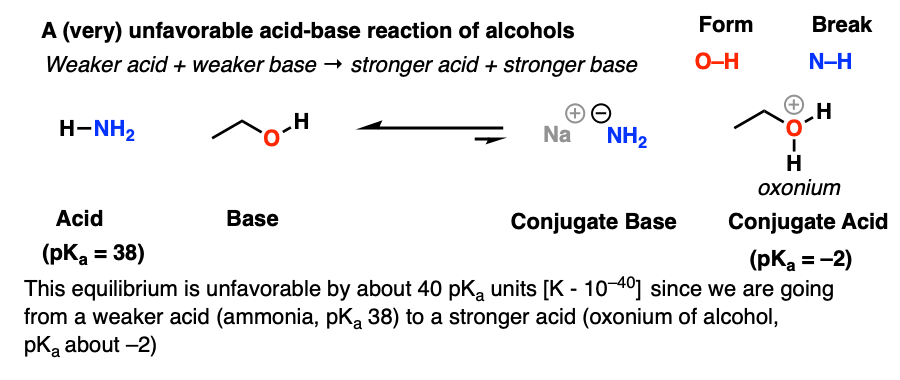Why is the conjugate acid of an ether/alcohol more acidic than that of a hydronium ion? : r/chemhelp

Mechanism and Kinetics of Isobutene Formation from Ethanol and Acetone over ZnxZryOz | ACS Catalysis

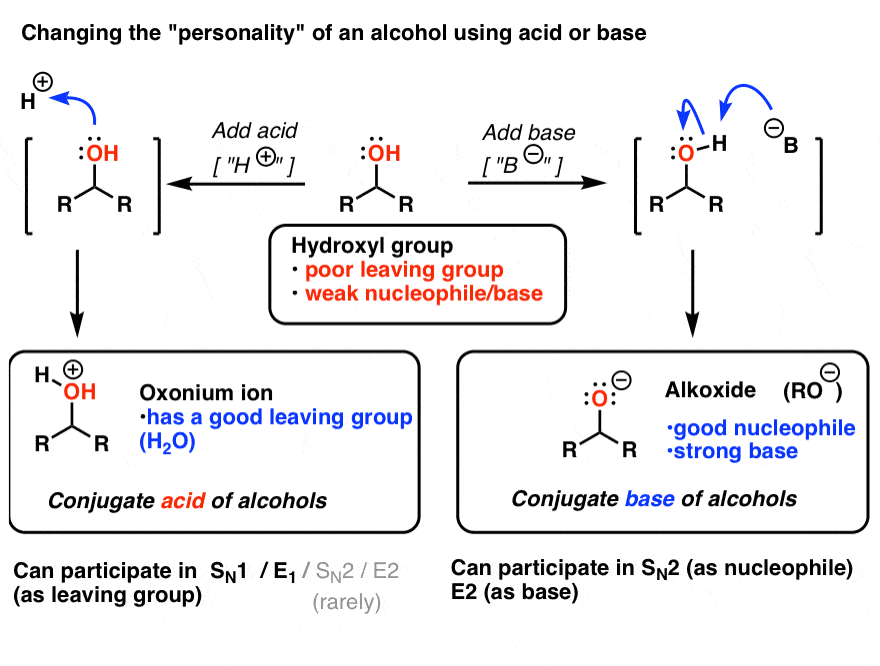
Draw the Lewis structure for the conjugate base from the reaction of ethanol with a generic base. Include all lone pairs of electrons and any nonzero formal charges. | Homework.Study.com
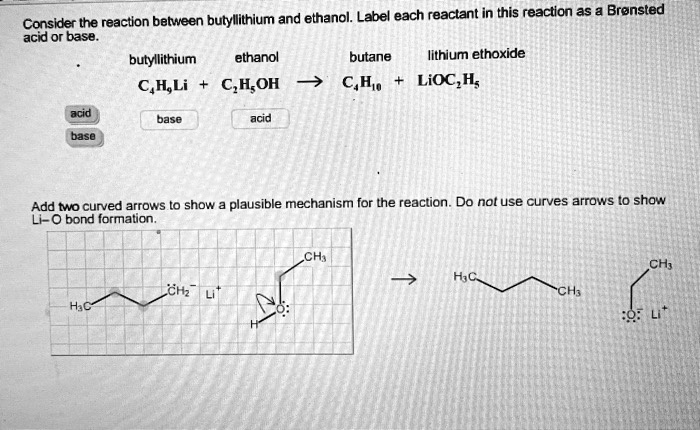
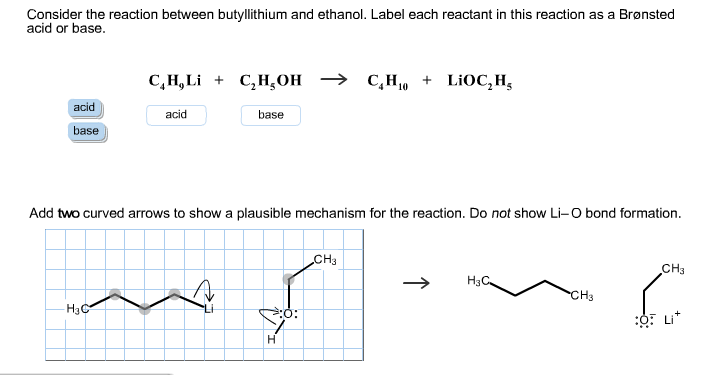
SOLVED: reaction belween butylithium and ethanol: Label each reactant in this reaction as Bronsted Consider the acid or base. butyllithium ethanol butane Ilthium ethoxide CH,Li C,H;OH C,H,o LiOC,Hs acid base acid base

Key Roles of Lewis Acid–Base Pairs on ZnxZryOz in Direct Ethanol/Acetone to Isobutene Conversion | Journal of the American Chemical Society

Influence of acid–base properties on the Lebedev ethanol-to-butadiene process catalyzed by SiO2–MgO materials - Catalysis Science & Technology (RSC Publishing)

Importance of the Nature of the Active Acid/Base Pairs of Hydroxyapatite Involved in the Catalytic Transformation of Ethanol to n‐Butanol Revealed by Operando DRIFTS - Osman - 2019 - ChemCatChem - Wiley Online Library